Autoimmune diseases arise when the immune system mistakenly attacks healthy tissue. Conditions such as rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, and Crohn’s disease reflect this immune misdirection. Adult stem cells have emerged as a scientifically grounded option for modifying this process rather than only suppressing symptoms.

The strongest clinical evidence centres on two adult stem cell types. Haematopoietic stem cells are blood-forming cells found in bone marrow and peripheral blood. In carefully selected patients with severe autoimmune disease, these cells have been used to “reset” immune function after intensive immune ablation. Long-term studies show sustained remission in subsets of patients with multiple sclerosis and systemic sclerosis.

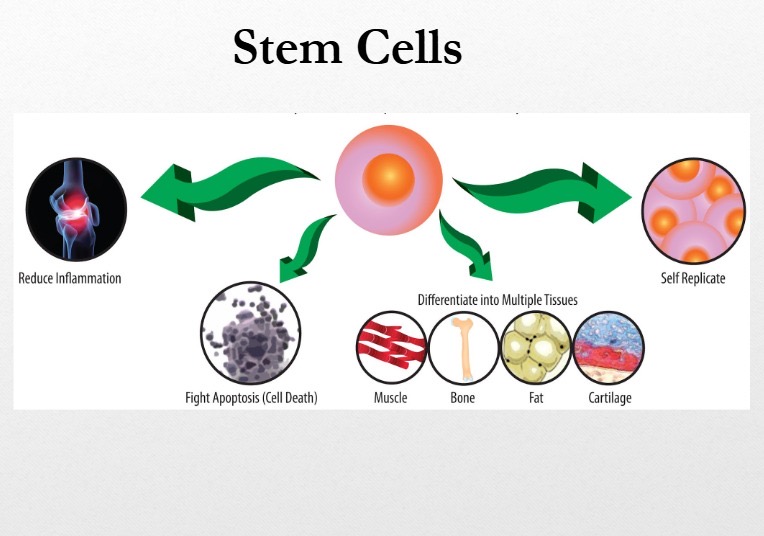
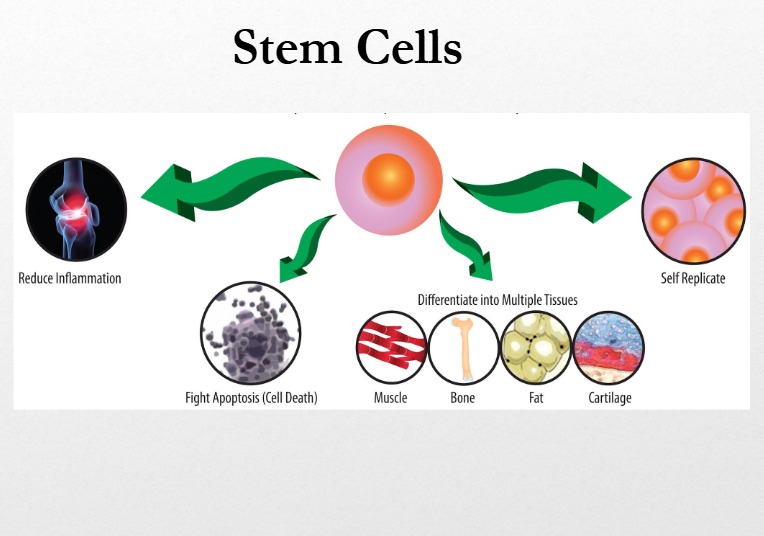
The second group, mesenchymal stromal cells (MSCs), are adult stem cells sourced from bone marrow, adipose tissue, and umbilical tissue. MSCs do not replace damaged organs. Instead, they regulate immune activity. They reduce harmful inflammation, promote regulatory immune cells that calm immune responses, and limit tissue injury. These effects explain their investigation in conditions driven by chronic immune activation.
Peer-reviewed clinical trials and meta-analyses show that MSCs can reduce disease activity and steroid dependence in disorders such as Crohn’s disease, graft-versus-host disease, and certain rheumatologic conditions. Safety data across thousands of treated patients show a favourable profile when cells are manufactured and administered under strict clinical standards.
Adult stem cell therapy does not cure autoimmune disease. It offers immune rebalancing in patients who have not responded to conventional treatment. Ongoing international trials continue to refine patient selection, dosing, and long-term outcomes, thereby positioning adult stem cells as a credible, evidence-based adjunct in modern autoimmune care.